

| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | ★ Recommendation | Older clinical validation; recent protocol |
| BP | BIHS (UK and IRL) | Self-measurement | Published evidence |
| BP | ESH (Europe) | Self-measurement | Published evidence |
| BP | Stride BP | Self-measurement for adults (Unavailable) (Previously Preferred) | Published evidence |
de Greeff A, Shennan AH. Validation of the Tensoval Duo Control II blood pressure monitor for clinic use and self-measurement according to the British Hypertension Society protocol and the European Society of Hypertension International Protocol Revision 2010. Blood Press Monit. 2013 Jun;18(3):161-6. doi: 10.1097/MBP.0b013e328360fb52. PMID: 23640067.
ESH-IP:2010 - Pass General population
Note: This is a provisional list, as equivalence according to EU Regulation 2017/745 (e.g. MEDDEV 2.7/1 rev 4) is not proven. Accordingly, these publications are not used in the assessment of star-ratings.
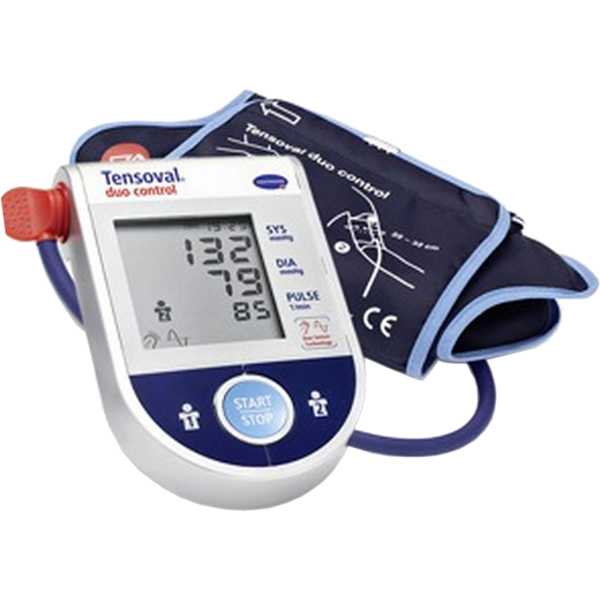
Hartmann Tensoval Duo Control
De Greeff A, Arora J, Hervey S, Liu B, Shennan AH. Accuracy assessment of the Tensoval duo control according to the British and European Hypertension Societies' standards. Blood Press Monit. 2008 Apr;13(2):111-6. doi: 10.1097/MBP.0b013e3282f3fb2e. PMID: 18347446.
ESH-IP:2002 - Pass General population
BHS:1993 - Pass (A/A) General population
de Greeff A, Hezelgrave N, Shennan AH. Accuracy assessment of a novel blood pressure measurement device in a South African adult population: Tensoval duo control. Blood Press Monit. 2011 Dec;16(6):304-6. doi: 10.1097/MBP.0b013e32834db648. PMID: 22027814.
ESH-IP:2002 General population (Note: ESH-02 not recognised since 01 July 2011)
Tholl U, Lüders S, Bramlage P, Dechend R, Eckert S, Mengden T, Nürnberger J, Sanner B, Anlauf M. The German Hypertension League (Deutsche Hochdruckliga) Quality Seal Protocol for blood pressure-measuring devices: 15-year experience and results from 105 devices for home blood pressure control. Blood Press Monit. 2016 Aug;22(4):197-205. doi: 10.1097/MBP.0000000000000186. PMID: 26998590.
DHL:1999 - Pass General population (Note: 2007)
DHL:2007 - Pass General population (Note: 2011, Normal and Large Cuff)