Integrity Applications GlucoTrack (NIBGM)
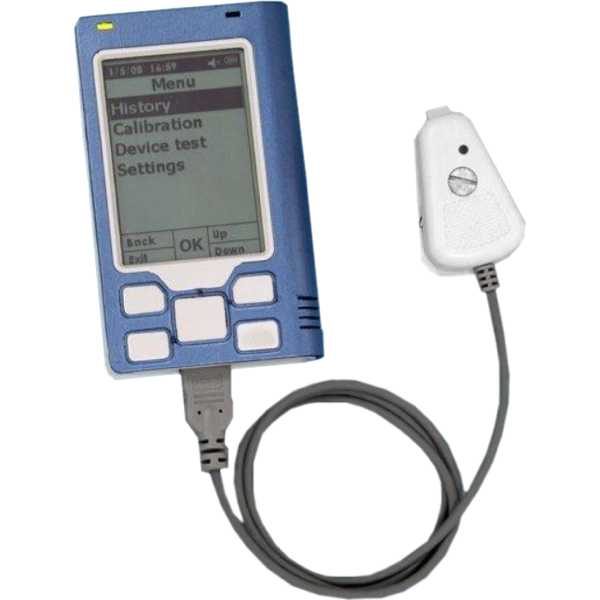
Device Name:
GlucoTrack (NIBGM)
Manufacturer:
Integrity Applications, 19 Ha’Yahalomim St., Ashdod, ISRAEL.
Measuring functions:
Blood glucose
Primary Client Use:
Intended for self-measurement and home use
Measurement Site:
Ear
Measurement Occurrence:
Intermittent measurements at specified intervals or times
Availability:
Available Currently
Description:
The Integrity Applications GlucoTrack (NIBGM) is an automatic blood glucose meter. Although its blood glucose measurement technology has been evaluated, it is not possible to base a determination on its accuracy from this research. Blood glucose measurements are taken from the ear. It is intended for self-measurement and home use.
Assessment:
The technology used in the Integrity Applications GlucoTrack (NIBGM), to measure blood glucose, has been assessed, in a general population, according to a non-standard protocol.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BG | Medaval | None | When an evaluation is carried out using a non-standard protocol, no inference can be made on accuracy based on a standard protocol. |
Validation Publications:
Harman-Boehm I, Gal A, Raykhman AM, Zahn JD, Naidis E, Mayzel Y. Noninvasive glucose monitoring: a novel approach. J Diabetes Sci Technol. 2010 Mar;3(2):253-60. Epub: 2009 Mar 1. doi: 10.1177/193229680900300205. PMID: 20144356. Available from: PMC2771521.
Ad Hoc protocol General population
Harman-Boehm I, Gal A, Raykhman AM, Naidis E, Mayzel Y. Noninvasive glucose monitoring: increasing accuracy by combination of multi-technology and multi-sensors. J Diabetes Sci Technol. 2010 May;4(3):583-595. Epub: 2010 May 1. doi: 10.1177/193229681000400312. PMID: 20513324. Available from: PMC2901035.
Ad Hoc protocol General population
