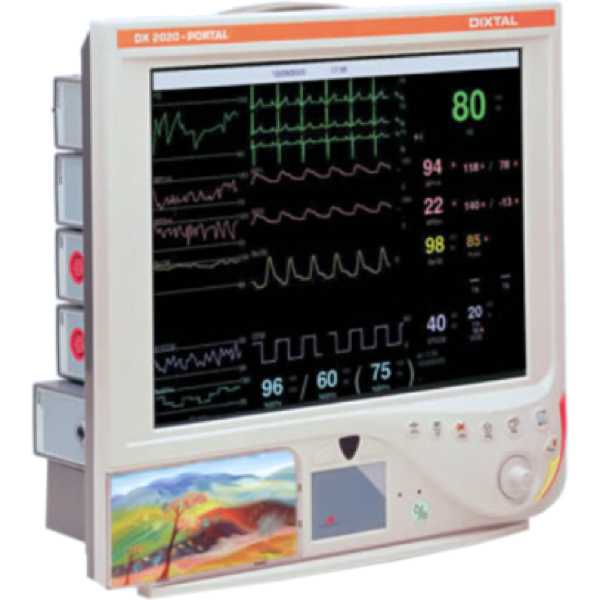
Dixtal DX-2020

Device Model:
DX-2020
Manufacturer:
Dixtal Medical Inc., 101 North Plains, Industrial Road Building, 2 Wallingford, CT 06492, UNITED STATES.
Measuring functions:
Blood Pressure and PR
Primary Client Use:
Intended for patient monitoring
Measurement Site:
Upper Arm
Measurement Occurrence:
Single measurements only
Availability:
Available Currently
Device Manual:
Description:
The Dixtal DX-2020 is a patient monitor. Medaval has not found evidence proving the accuracy of its blood pressure measurement technology. Blood pressure measurements are taken from the upper arm. It is intended for bedside patient monitoring.
Assessment:
The technology used in the Dixtal DX-2020, to measure blood pressure, has failed in a clinical validation study, in a general population, according to a recognised standard protocol, as published in a peer-reviewed publication.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | Not recommended | This device has failed a clinical validation. |
Validation Publications:
Gothardo AC, Savioli AF, Santos DS, Lamas JL. Lack of validation of the Dixtal (DX 2020) upper arm blood pressure monitor, in oscillometric mode, for clinical use in an intensive care unit, according to the European Society of Hypertension-International Protocol revision 2010. Blood Press Monit. 2013 Aug;18(4):215-8. doi: 10.1097/MBP.0b013e3283624ad7. PMID: 23695347.
ESH-IP:2010 - Fail General population
