Spengler AutoTensio Arm (SPG440)
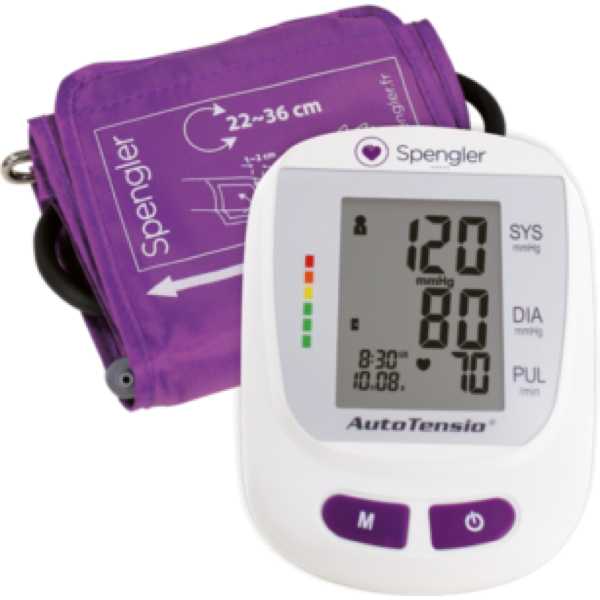

Device Name:
AutoTensio
Device Model:
SPG440
Manufacturer:
Spengler S.a.s., 190 rue Paul Langevin, ZAC La Robole, 13856 Aix-en-Provence, FRANCE.
Measuring functions:
Blood Pressure and PR
Primary Client Use:
Intended for self-measurement and home use
Measurement Site:
Upper Arm
Measurement Occurrence:
Intermittent measurements at specified intervals or times
Availability:
Available Currently
Description:
The Spengler AutoTensio Arm (SPG440) is an automatic blood pressure monitor. Medaval has not found evidence proving the accuracy of its blood pressure measurement technology. Blood pressure measurements are taken from the upper arm. It is intended for self-measurement and home use.
Assessment:
There appears to be no peer-reviewed clinical validation information available on the technology used in the Spengler AutoTensio Arm (SPG440) to measure blood pressure.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | Not recommended | This device has not been clinically validated. |
Device Family:
Aspen BP-10 (BP-103H)Z, Beurer BM 26P, Beurer BM 40P, Beurer BM 35/1D,P, Braun BP-4900D, Braun BP-5900D, Healthcare World BP-103HZ, Hartmann Veroval 2-in-1 ECG (BP750X)P, HyMark DBP-1314 (BP-103H)Z, LotFancy BP-103HZ, LotFancy BP-1305Z, Sejoy Joytech DBP-1204 (Sejoy BP-102)F, Sejoy Joytech DBP-1314 (Sejoy BP-103H)F, Sejoy Joytech DBP-1209 (Sejoy BP-1209)F, Sejoy Joytech DBP-1303 (Sejoy BP-1303)F, Sejoy Joytech DBP-1305 (Sejoy BP-1305, Joytech DBP-1305)F, Sejoy Joytech DBP-1307 (Sejoy BP-1307, Joytech DBP-1307)B,P,F, Sejoy Joytech DBP-1231F, Sejoy Joytech DBP-1332F, Sejoy Joytech DBP-1334F, Spengler AutoTensio Arm (SPG440)P, Spengler Tensonic ArmP, Xuerui XR-BP-102Z, Xuerui XR-BP-103HZ, Xuerui XR-BP-1209Z, Xuerui XR-BP-1303Z, Xuerui XR-BP-1305Z, Xuerui XR-BP-1307Z
Legend: B BIHS Derivative, D DET Equivalence, P Stride Equivalence, F FDA 510(k), Z Almost Certain
Legend: B BIHS Derivative, D DET Equivalence, P Stride Equivalence, F FDA 510(k), Z Almost Certain
Validation Publications for Equivalent Devices:
Note: This is a provisional list, as equivalence according to EU Regulation 2017/745 (e.g. MEDDEV 2.7/1 rev 4) is not proven. Accordingly, these publications are not used in the assessment of star-ratings.
Sejoy Joytech DBP-1307 (Sejoy BP-1307, Joytech DBP-1307)
Lei L, Chen Y, Chen Q, Li Y, Wang JG. Validation of the SEJOY BP-1307 upper-arm blood pressure monitor for home blood pressure monitoring according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2017 Dec;22(6):371-4. Epub: 2017 Aug 03. doi: 10.1097/MBP.0000000000000283. PMID: 28786853 .
ESH-IP:2010 - Pass General population
