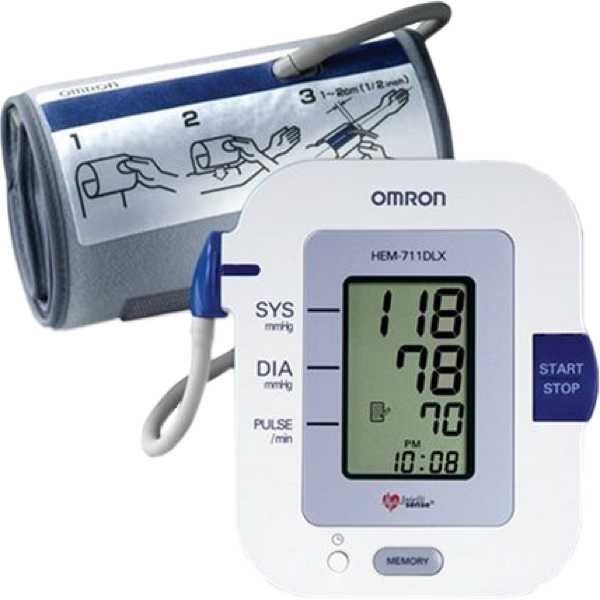
Omron HEM-711DLX

Device Model:
HEM-711DLX
Manufacturer:
Omron Corporation, Kyoto Head Office, Shiokoji Horikawa, Shimogyo ku, Kyoto 600-8530, JAPAN.
Measuring functions:
Blood pressure
Primary Client Use:
Intended for both professional use and self-measurement
Measurement Site:
Upper Arm
Measurement Occurrence:
Single measurements only
Availability:
Discontinued but still available
Device Manual:
Description:
The Omron HEM-711DLX is an automatic blood pressure monitor. Its blood pressure measurement technology has been proven to be accurate. Blood pressure measurements are taken from the upper arm. It is intended for both professional use and self-measurement. This device has been discontinued by the manufacturer but may be available through certain outlets.
Assessment:
The technology used in the Omron HEM-711DLX, to measure blood pressure, has passed in a clinical validation study, in a general population, according to a recognised standard protocol, as published in a peer-reviewed publication.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | Legacy approval | Older clinical validation; older protocol |
| BP | BIHS (UK and IRL) | Previous recommendation, now archived (Archived) | Published evidence |
| BP | ESH (Europe) | Self-measurement and professional use | Published evidence |
| BP | Stride BP | Self-measurement for adults (Unavailable) | Published evidence |
Validation Publications:
Grim CE, Grim CM. Omron HEM-711 DLX home Blood pressure monitor passes the European Society of Hypertension International Validation Protocol. Blood Press Monit. 2008 Aug;13(4):225-6. doi: 10.1097/MBP.0b013e3282feebd5. PMID: 18635978.
ESH-IP:2002 - Pass General population
