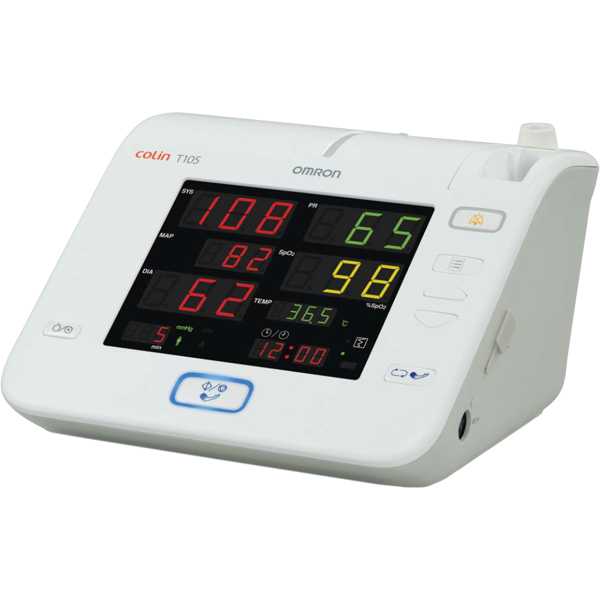
Omron HBP-T105
Device Name:
Colin HBP-T105
Device Model:
HBP-T105
Manufacturer:
Omron Corporation, Kyoto Head Office, Shiokoji Horikawa, Shimogyo ku, Kyoto 600-8530, JAPAN.
Measuring functions:
Blood pressure, SpO2, Temperature
Primary Client Use:
Intended for patient monitoring
Measurement Site:
BP: Upper Arm; SpO2: Finger
Measurement Occurrence:
BP & SpO2: Single and intermittent measurements
Availability:
Discontinued but still available
Device Manual:
Device Specifications:
Description:
The Omron HBP-T105 is a patient monitor. Its blood pressure measurement technology has been proven to be accurate and information regarding the accuracy of its oxygen saturation measurement technology does not appear to be available. Blood pressure measurements are taken from the upper arm and oxygen saturation measurements are taken from the finger. It is intended for bedside patient monitoring. This device has been discontinued by the manufacturer but may be available through certain outlets.
Assessment:
The technology used in the Omron HBP-T105, to measure blood pressure, has passed in a clinical validation study, in a general population, according to a recognised standard protocol, as published in a peer-reviewed publication. There appears to be no peer-reviewed clinical validation information available on the technology used in the Omron HBP-T105 to measure peripheral oxygen saturation.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | Legacy approval | Older clinical validation; older protocol |
| BP | Stride BP | Office and hospital use for adults (Unavailable) | Published evidence |
Device Family:
Validation Publications:
Asmar R, Khabouth J, Mattar J, Pecchioli V, Germano G. Validation of three professional devices measuring office blood pressure according to three different methods: the Omron BP10, the Omron HBP T105 and the Pic Indolor Professional. J Hypertens. 2010 Mar;28(3):452-8. doi: 10.1097/HJH.0b013e3283340c2b. PMID: 20087217.
ESH-IP:2002 - Pass General population
