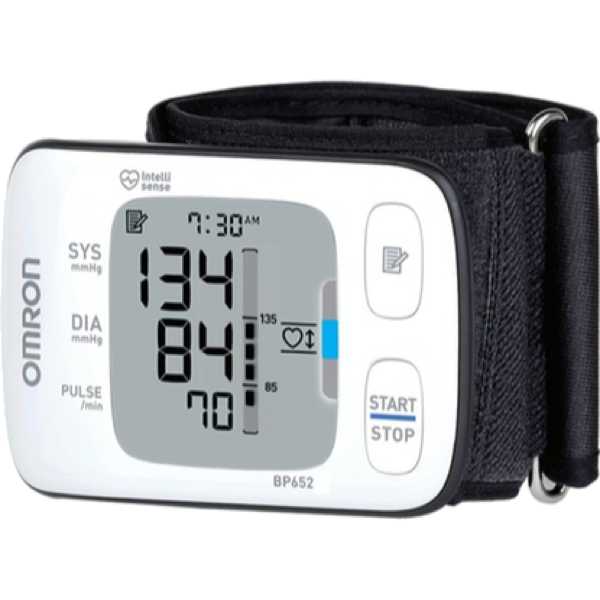
Omron BP652N (HEM-6300-Z)
Device Name:
BP652N
Device Model:
HEM-6300-Z
Manufacturer:
Omron Corporation, Kyoto Head Office, Shiokoji Horikawa, Shimogyo ku, Kyoto 600-8530, JAPAN.
Measuring functions:
Blood Pressure and PR
Primary Client Use:
Intended for self-measurement and home use
Measurement Site:
Wrist
Measurement Occurrence:
Single measurements only
Availability:
Available Currently
Availability according to Countries or Regions:
United States
Device Manual:
Description:
The Omron BP652N (HEM-6300-Z) is an automatic blood pressure monitor. The accuracy of its blood pressure measurement technology has yet to be proven to MDR requirements. Blood pressure measurements are taken from the wrist. It is intended for self-measurement and home use.
Assessment:
The technology used in the Omron BP652N (HEM-6300-Z), to measure blood pressure, has been declared as being substantially equivalent to that used in another device, according to 510(k), but this is not compliant with (EU) 2017/745 and MEDDEV 2.7/1 rev 4.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | None | Although equivalence to another device is claimed, this has not been tested to MDR requirements. |
| BP | FDA (US) | 510(k) | 510(k) K123498, and, K142917 |
| Consumer Body | Year(s) | Level | Comment | |
| BP | Consumer Reports (US) | Listed but not as current: 2018-2025 | Very Good | |
| BP | Amazon Best Sellers (US) | Latest: 2019 | B (3.6 / 5) |
Device Family:
Omron HEM-6131F, Omron BP652N (HEM-6300-Z)F, Omron HeartGuide (HEM-6411T-MAE)P, Omron Heartguide BP8000-M (HEM-6410T-ZM)P,F, Omron BP4350 (HEM-6232T-Z) GoldF
Legend: P Stride Equivalence, F FDA 510(k)
Legend: P Stride Equivalence, F FDA 510(k)
Validation Publications for Equivalent Devices:
Note: This is a provisional list, as equivalence according to EU Regulation 2017/745 (e.g. MEDDEV 2.7/1 rev 4) is not proven. Accordingly, these publications are not used in the assessment of star-ratings.
Omron Heartguide BP8000-M (HEM-6410T-ZM)
Kuwabara M, Harada K, Hishiki Y, Kario K. Validation of two watch-type wearable blood pressure monitors according to the ANSI/AAMI/ISO81060-2:2013 guidelines: Omron HEM-6410T-ZM and HEM-6410T-ZL. J Clin Hypertens (Greenwich). 2019 Jun;21(6):853-858. Epub: 2019 Feb 25. doi: 10.1111/jch.13499. PMID: 30803128.
81060-2:2013 - Pass General population