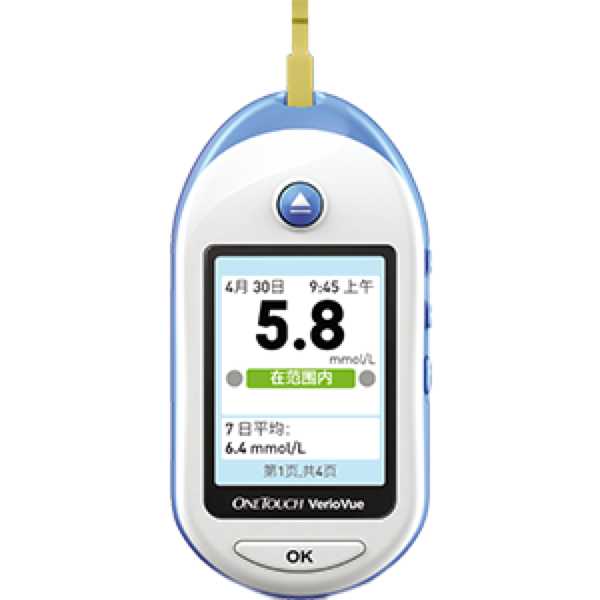
LifeScan OneTouch Verio Vue

Device Name:
OneTouch Verio Vue
Manufacturer:
Johnson & Johnson, 1 Johnson & Johnson Plaza, New Brunswick, New Jersey 08933, UNITED STATES.
Measuring functions:
Blood glucose
Primary Client Use:
Intended for self-measurement and home use
Measurement Site:
Finger
Measurement Occurrence:
Single measurements only
Availability:
Discontinued but still available
Description:
The LifeScan OneTouch Verio Vue is an automatic blood glucose meter. Medaval has not found evidence proving the accuracy of its blood glucose measurement technology. Blood glucose measurements are taken from the finger. It is intended for self-measurement and home use. This device has been discontinued by the manufacturer but may be available through certain outlets.
Assessment:
There appears to be no peer-reviewed clinical validation information available on the technology used in the LifeScan OneTouch Verio Vue to measure blood glucose.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BG | Medaval | Not recommended | This device has not been clinically validated. |
Relevant Publications:
Katz LB, Grady M, Setford J, Levy BL. OneTouch Blood Glucose Monitoring Systems – Impact of New Technologies on the Efficacy of Self-monitoring Blood Glucose US Endocrinology. 2018 Jan;14(Suppl 1):2-8. Epub: 2018 Jan 22. Available from: www.touchendocrinology.com.
Given the struggles that many patients have with ongoing adherence, motivation, health literacy and/or numeracy, the newer BGMS features described in this article represent important tools which may help HCPs better communicate with their patients on how to both understand their BG data and encourage appropriate self-management from their patients.
