| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | None | Although equivalence to another device is claimed, this has not been tested to MDR requirements. |
| BP | Hypertension Canada | Public use (Gold) | Manufacturer declaration of equivalence |
| BP | Stride BP | Public use for adults (Preferred) | Equivalence to the InBody BPBIO320 claim without published evidence |
Legend: B BIHS Derivative, P Stride Equivalence
Moon JH, Kang MK, Choi CE, Min J, Lee HY, Lim S. Validation of a wearable cuff-less wristwatch-type blood pressure monitoring device. Sci Rep. 2020 Nov 4;10(1):19015. doi: 10.1038/s41598-020-75892-y. PMID: 33149118. Available from: PMC7642418.
Ad Hoc protocol General population
Note: This is a provisional list, as equivalence according to EU Regulation 2017/745 (e.g. MEDDEV 2.7/1 rev 4) is not proven. Accordingly, these publications are not used in the assessment of star-ratings.
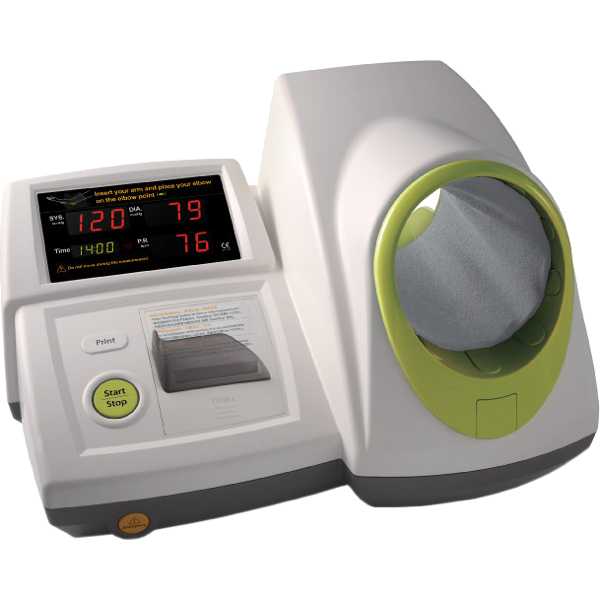
InBody BPBIO320
Kollias A, Stambolliu E, Kyriakoulis KG, Papadatos SS, Stergiou GS. Validation of the single-cuff oscillometric blood pressure monitor InBody BPBIO320 for public use according to the 2010 European Society of Hypertension International Protocol. Blood Press Monit. 2019 Feb;24(1):30-32. Epub: 2018 Dec 7. doi: 10.1097/MBP.0000000000000359. PMID: 30531495.
ESH-IP:2010 - Pass General population
InBody BPBIO750
Ntineri A, Prapa S, Bountzona I, Menti A, Stergiou GS. Validation of the single-cuff oscillometric blood pressure monitor InBody BPBIO750 for public spaces according to the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization Universal Standard. Blood Press Monit. 2021 Apr 1;26(2):146-148. Epub: 2020 Dec 14. doi: 10.1097/MBP.0000000000000507. PMID: 33323723.
81060-2:2018 - Pass General population