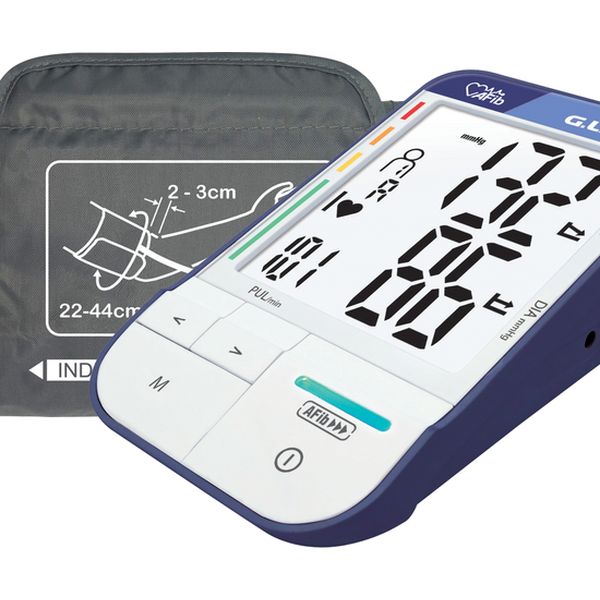
OBL: G.LAB, Zhukeng Community, Pingshan New District, Shenzhen, Guangdong Province, CHINA.
OEM: Grandway Healthcare Ltd., Room 1705, Kinox Centre, No. 9 Hung To Road, Kwun Tong, Kowloon, HONG KONG PRC.
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | Recommended for pregnancy only | Validation in pregnancy |
| BP | Stride BP | Self-measurement for diabetes mellitus and pregnancy (Preferred) | Published evidence |
Li LY, Tian L, Gao B, Ming J, Liu K, Jiang J, Han ZY, Zhao LY, Chen CS, Wan Y. Validation of the G.LAB MD41A0 upper arm blood pressure monitor in patients with diabetes mellitus according to the AAMI/ESH/ISO 81060-2: 2018 Universal Standard. Blood Press Monit. 2022 Aug 1;27(4):280-284. Epub: 2022 Mar 7. doi: 10.1097/MBP.0000000000000595.
81060-2:2019 - Pass Type 2 Diabetes Mellitus
Zhang Y, Shi Z, Liang H, Chen M, Sun Y, Shang F, Wan Y. Clinical Accuracy of the G.LAB MD41A0 Upper Arm Blood Pressure Monitor in Pregnancy and Pre-Eclampsia Using the AAMI/ESH/ISO as Reference Standard. Vasc Health Risk Manag. 2024 Nov 3;20:487-492. doi: 10.2147/VHRM.S479380. PMID: 39524156. Available from: PMC11549012.
81060-2:2019 - Pass Normotensive pregnancy (n=15), Hypertensive pregnancy (n=15), Pre-eclampsia (n=15) (Note: Overall results.)
