Fukuda Denshi DS-1200

Device Name:
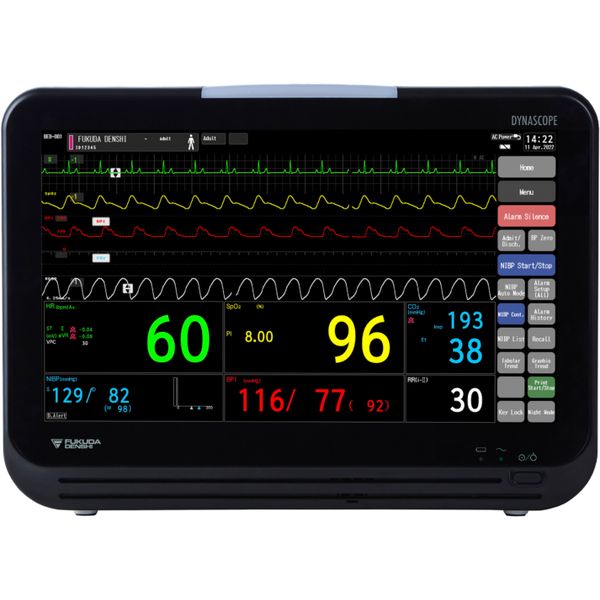
Dynascope
Device Model:
DS-1200
Manufacturer:
Fukuda Denshi Co. Ltd., 2-23-5 Kita Ueno, Taito-Ku, Tokyo 110-0014, JAPAN.
Measuring functions:
Blood Pressure, SpO2 and PR
Primary Client Use:
Intended for patient monitoring
Measurement Site:
BP: Upper Arm; SpO2: Finger
Measurement Occurrence:
BP & SpO2: Intermittent measurements at specified intervals or times
Availability:
Available Currently
Availability according to Countries or Regions:
Japan
Description:
The Fukuda Denshi DS-1200 is a patient monitor. Medaval has not found evidence proving the accuracy of its blood pressure measurement technology and information regarding the accuracy of its oxygen saturation measurement technology does not appear to be available. Blood pressure measurements are taken from the upper arm and oxygen saturation measurements are taken from the finger. It is intended for bedside patient monitoring.
Assessment:
While the technology used in the Fukuda Denshi DS-1200, to measure blood pressure, has been declared as being equivalent to that used in another device, not only has no evidence has been published to show that the devices have been compared according to a protocol compliant with (EU) 2017/745 and MEDDEV 2.7/1 rev 4, but there is no evidence provided on a validation of the other device. There appears to be no peer-reviewed clinical validation information available on the technology used in the Fukuda Denshi DS-1200 to measure peripheral oxygen saturation.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | Not recommended | This device has not been shown to have been clinically validated. |
| BP | Japanese Society of Hypertension | Professional use (2022-2024) | Implied equivalence to the Fukuda Denshi NIBP-771 claim without published evidence |
Device Family:
Fukuda Denshi FORM-5J, Fukuda Denshi SC-1800 (COL-SC-1800)J, Fukuda Denshi DS-1200J, Fukuda Denshi BDS-1001J
Legend: J JHS Implied Equivalence
Legend: J JHS Implied Equivalence
