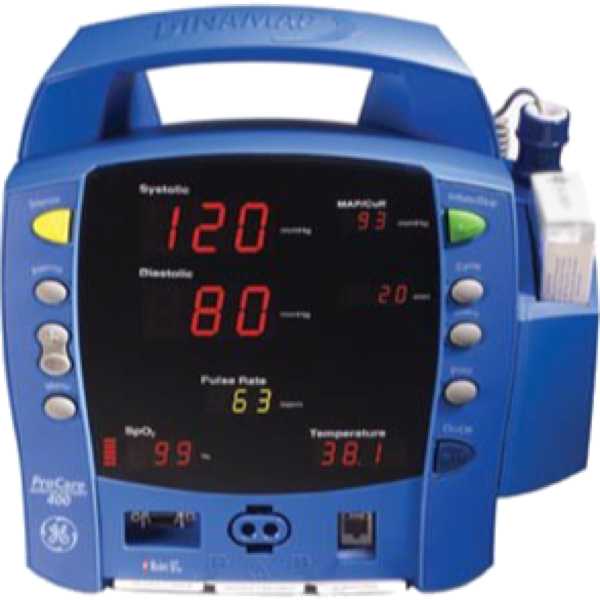
Dinamap ProCare 400
Device Name:
ProCare 400
Manufacturer:
GE Medical Systems Ltd. (Dinamap), Amersham Place, Little Chalfont, Buckinghamshire HP7 9NA, UNITED KINGDOM.
Measuring functions:
Blood pressure
Primary Client Use:
Intended for patient monitoring
Measurement Site:
Upper Arm
Measurement Occurrence:
Intermittent measurements at specified intervals or times
Availability:
Available Currently
Device Manual:
Description:
The Dinamap ProCare 400 is a patient monitor. Its blood pressure measurement technology has been proven to be accurate. Blood pressure measurements are taken from the upper arm. It is intended for bedside patient monitoring.
Assessment:
The technology used in the Dinamap ProCare 400, to measure blood pressure, has passed in two clinical validation studies, in pregnancy populations, according to recognised standard protocols, as published in peer-reviewed publications.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | Recommended for pregnancy only | Validation in pregnancy |
| BP | ESH (Europe) | Professional use for pregnancy | A BIHS recommendation that does not exist |
| BP | Stride BP | Office and hospital use for pregnancy (Previously Preferred) | Published evidence |
| BP | FDA (US) | 510(k) | 510(k) K014255 |
Validation Publications:
de Greeff A, Ghosh D, Anthony J, Shennan A. Accuracy assessment of the Dinamap ProCare 400 in pregnancy and preeclampsia. Hypertens Pregnancy. 2010 Jan;29(2):198-205. doi: 10.3109/10641950902968650. PMID: 20367507.
BHS:1993 - Pass (A/A) Pregnancy
BHS:1993 - Pass (A/B) Pre-eclampsia
Relevant Publications:
Brothwell S, Dutton M, Ferro C, Stringer S, Cockwell P. Optimising the accuracy of blood pressure monitoring in chronic kidney disease: the utility of BpTRU. BMC Nephrol. 2013 Oct 10;14:218. doi: 10.1186/1471-2369-14-218. PMID: 24112304. Available from: PMC3852944.
Measurements from the Dinamap ProCare 400 are compared to those from the BPM-100 and the ABPM-04, though not in a formal validation.
