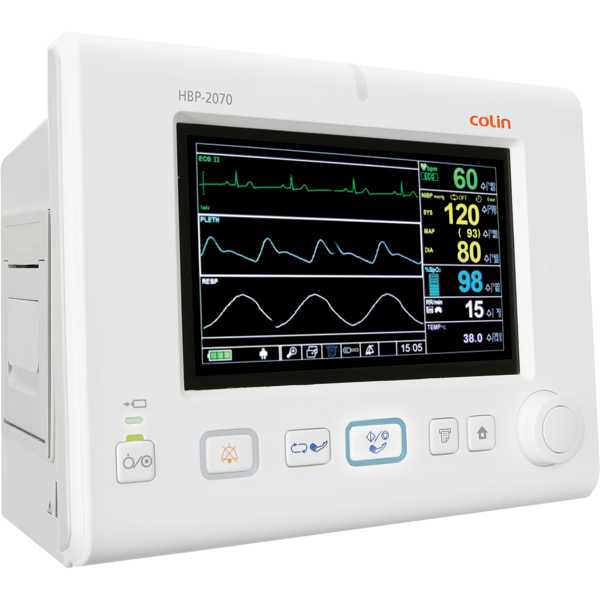
Device Model:
HBP-2070
Manufacturer:
Colin Medical Instruments Corp., 5850 Farinon Dr, San Antonio, TX 78249, UNITED STATES.
Measuring functions:
Blood Pressure, SpO2, PR, ECG, Temperature and Respiration
Primary Client Use:
Intended for patient monitoring
Measurement Site:
BP: Upper Arm; SpO2: Finger
Measurement Occurrence:
BP: Single, intermittent and continuous measurements; SpO2: Continuous measurement
Availability:
Available Currently
Availability according to Countries or Regions:
Japan
Device Manual:
Description:
The Colin HBP-2070 is a vital signs monitor. The accuracy of its blood pressure measurement technology has yet to be proven to MDR requirements and information regarding the accuracy of its oxygen saturation measurement technology does not appear to be available. Blood pressure measurements are taken from the upper arm and oxygen saturation measurements are taken from the finger. It is intended for bedside patient monitoring.
Assessment:
The technology used in the Colin HBP-2070, to measure blood pressure, has been compared to a clinically validated device, but the results of the equivalence study have not been published and cannot be verified; nor can the protocol be checked for compliance with (EU) 2017/745 and MEDDEV 2.7/1 rev 4. There appears to be no peer-reviewed clinical validation information available on the technology used in the Colin HBP-2070 to measure peripheral oxygen saturation.
Recommendations:
| Accuracy Assessment | Recommendation | Basis |
| BP |
Medaval |
None |
Although equivalence to another device is claimed, this has not been tested to MDR requirements. |
| BP |
Japanese Society of Hypertension |
Professional use (Previous: 2016-2018) |
Implied equivalence to the Omron M3500 NIBP Module |
Device Family:
Validation Publications for Equivalent Devices:
Note: This is a provisional list, as equivalence according to EU Regulation 2017/745 (e.g. MEDDEV 2.7/1 rev 4) is not proven. Accordingly, these publications are not used in the assessment of star-ratings.
Omron M3500 NIBP Module
Chahine MN, Assemaani N, Sayed Hassan G, Cham M, Salameh P, Asmar R. Validation of the OMRON M3500 Blood Pressure Measuring Device Using Normal- and High-Speed Modes in Adult and Specific Populations (Obese and Children) According to AAMI Protocol. J Clin Hypertens (Greenwich). 2015 Aug;17(8):622-9. Epub: 2015 Apr 2. doi: 10.1111/jch.12540.. PMID: 25833259. Available from: onlinelibrary.wiley.com.
81060-2:2009 - Pass In normal-speed mode (Note: 81060-2:2013 criteria fulfilled)
81060-2:2009 - Pass In high-speed mode (Note: 81060-2:2013 criteria fulfilled)