Cofoe KF-65B
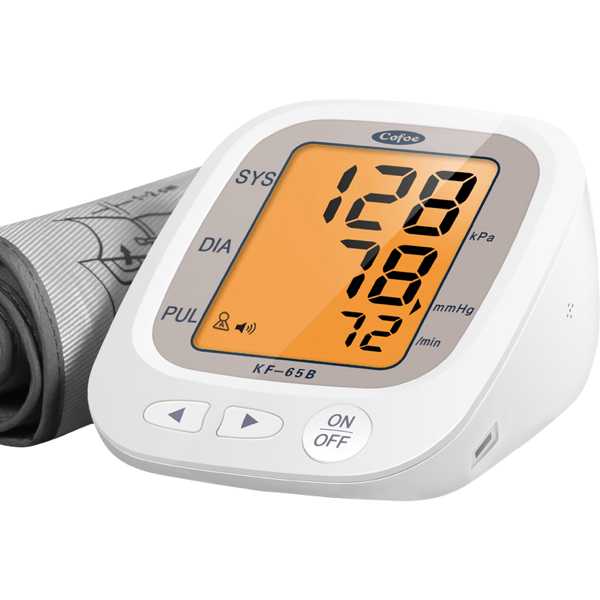

Device Model:
KF-65B
Manufacturer:
Cofoe Medical Technology Co. Ltd., 816 Zhenhua Road, Yuhua District, Changsha, Hunan 410000, CHINA.
Measuring functions:
Blood pressure
Primary Client Use:
Intended for self-measurement and home use
Measurement Site:
Upper Arm
Measurement Occurrence:
Single measurements only
Connected Health Technologies:
Bluetooth
Availability:
Available Currently
Description:
The Cofoe KF-65B is an automatic blood pressure monitor. Medaval has not found evidence proving the accuracy of its blood pressure measurement technology. Blood pressure measurements are taken from the upper arm. It is intended for self-measurement and home use.
Assessment:
The technology used in the Cofoe KF-65B, to measure blood pressure, has failed in a clinical validation study, in a general population, according to a recognised standard protocol, as published in a peer-reviewed publication.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | Not recommended | This device has failed a clinical validation. |
| BP | BIHS (UK and IRL) | NOT recommended (Previous) | Published evidence |
Validation Publications:
Zhang P, Li X, Fang Z, Lu Y, Cui J, Du X, Hu R. Smartphone application-supported validation of three automatic devices for self-measurement of blood pressure according to the European Society of Hypertension International Protocol revision 2010: the Omron HEM-7120, Yuwell YE680A and Cofoe KF-65B. Blood Press Monit. 2021 Dec 1;26(6):435-440. Epub: 2021 May 13. doi: 10.1097/MBP.0000000000000547. PMID: 34001755.
ESH-IP:2010 - Fail General population
