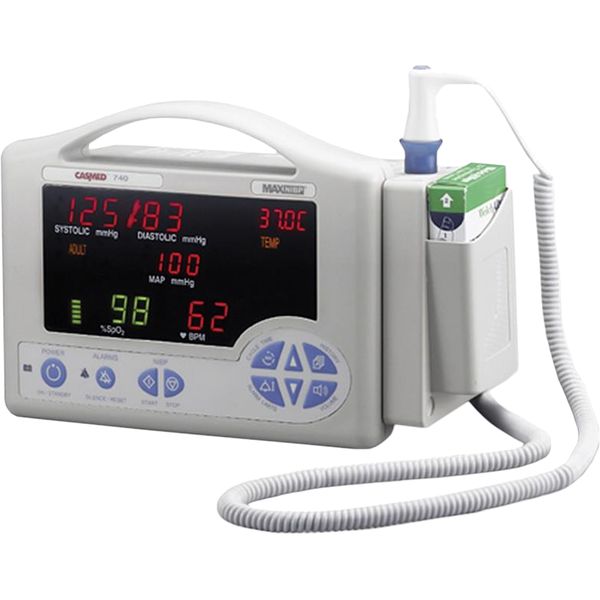
CasMed 740
Device Name:
740
Manufacturer:
CAS Medical Systems Inc., 44 East Industrial Road, Branford, CT 06405, UNITED STATES.
Measuring functions:
Blood pressure, SpO2, Temperature
Primary Client Use:
Intended for patient monitoring
Measurement Site:
BP: Upper Arm; SpO2: Finger
Measurement Occurrence:
BP & SpO2: Intermittent measurements at specified intervals or times
Availability:
Available Currently
Device Specifications:
Description:
The CasMed 740 is a vital signs monitor. Its blood pressure measurement technology has been proven to be accurate and information regarding the accuracy of its oxygen saturation measurement technology does not appear to be available. Blood pressure measurements are taken from the upper arm and oxygen saturation measurements are taken from the finger. It is intended for bedside patient monitoring.
Assessment:
The technology used in the CasMed 740, to measure blood pressure, has passed in a clinical validation study, in a young population, according to a recognised standard protocol, as published in a peer-reviewed publication. There appears to be no peer-reviewed clinical validation information available on the technology used in the CasMed 740 to measure peripheral oxygen saturation.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | Recommended for children only | Validation in children |
| BP | ESH (Europe) | Professional use for children | Published evidence |
Validation Publications:
Lang SM, Giuliano JS Jr, Carroll CL, Rosenkrantz TS, Eisenfeld L. Neonatal/infant validation study of the CAS model 740 noninvasive blood pressure monitor with the Orion/MaxIQ NIBP module. Blood Press Monit. 2014 Jun;19(3):180-2. doi: 10.1097/MBP.0000000000000036. PMID: 24618916.
81060-2:2009 - Pass Neonates and infants (n=29)
