OBL: Beurer GmbH, Söflinger Straße 218, 89077 Ulm, GERMANY.
OEM: Globalcare Medical Technology Co. Ltd., Building 7, 39 Middle Industrial Main Road, Xiaolan Industrial Zone, Xiaolan Town, Zhongshan City, Guangdong 528415, CHINA.
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | None | Although equivalence to another device is claimed, this has not been tested to MDR requirements. |
Legend: P Stride Equivalence, Z Almost Certain
Note: This is a provisional list, as equivalence according to EU Regulation 2017/745 (e.g. MEDDEV 2.7/1 rev 4) is not proven. Accordingly, these publications are not used in the assessment of star-ratings.
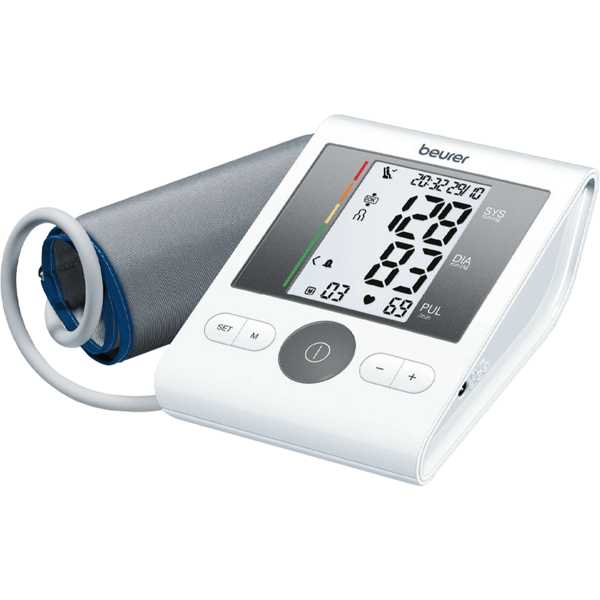
Beurer BM 28
Deutsch C, Bramlage C, Botta B, Krüger R, Forstner K, Bramlage P, Beime B. Validation of the blood pressure measurement device Beurer BM 28 according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2021 Aug 1;26(4):292-298. Epub: 2021 Mar 18. doi: 10.1097/MBP.0000000000000528. PMID: 33741775.
ESH-IP:2010 - Pass General population
Globalcare GCE603
Song C, Yu Y, Lu BC, Yan XL. Validation of the Globalcare GCE603 automated blood pressure monitor for self-measurement according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2020 Oct;25(5):291-294. Epub: 2020 Jun 23. doi: doi: 10.1097/MBP.0000000000000461. PMID: 32898351.
ESH-IP:2010 - Pass General population
