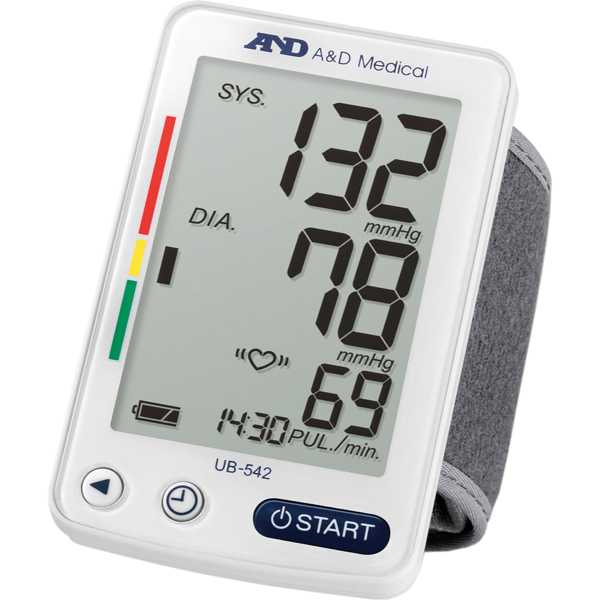
A&D UB-542

Device Model:
UB-542
Manufacturer:
A&D Company Ltd., 3-23-14 Higashi-Ikebukuro, Toshima-Ku, 170-0013 Tokyo, JAPAN.
Measuring functions:
Blood pressure
Primary Client Use:
Intended for self-measurement and home use
Measurement Site:
Wrist
Measurement Occurrence:
Single measurements only
Availability:
Available Currently
Availability according to Countries or Regions:
Australia
Device Manual:
Description:
The A&D UB-542 is an automatic blood pressure monitor. Its blood pressure measurement technology has been proven to be accurate, with a 1-star Medaval rating. Blood pressure measurements are taken from the wrist. It is intended for self-measurement and home use.
Assessment:
The technology used in the A&D UB-542, to measure blood pressure, has passed in a clinical validation study, in a general population, according to a recognised standard protocol, as published in a peer-reviewed publication.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | ★ Recommendation | Older clinical validation; recent protocol |
| BP | BIHS (UK and IRL) | Self-measurement | Published evidence |
| Consumer Body | Year(s) | Level | Comment | |
| BP | Choice (Australia) |
Validation Publications:
Saladini F, Benetti E, Fania C, Palatini P. Validation of the A&D BP UB-542 wrist device for home blood pressure measurement according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2013 Aug;18(4):219-22. doi: 10.1097/MBP.0b013e3283624aa2. PMID: 23681205.
ESH-IP:2010 - Pass General population