
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | ★★ Recommendation | Recent clinical validation; recent protocol |
| BP | Japanese Society of Hypertension | Self-measurement (Previous: 2022-2023) | Unpublished validation |
| BP | Stride BP | Self-measurement for adults | Equivalence to the A&D UA-1020 claim without published evidence |
Legend: L AHA VDL Implied Equivalence, B BIHS Derivative, J JHS Implied Equivalence, P Stride Equivalence, Z Almost Certain
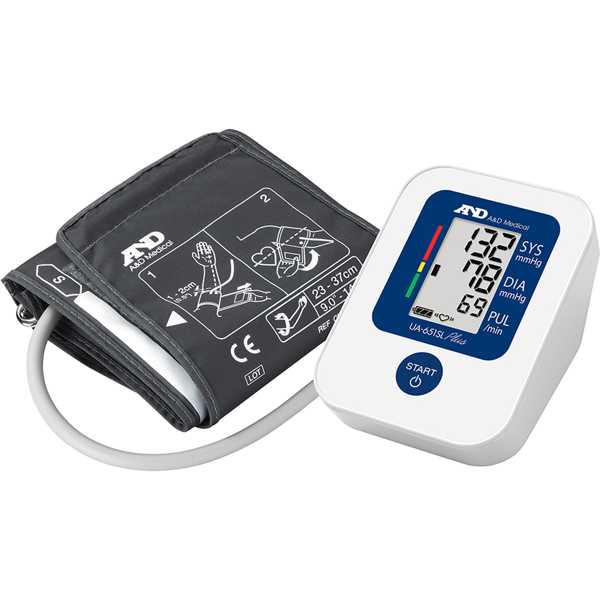
Alpert BS. Validation of the A&D UA-651 Plus/UA-651SL Plus automated sphygmomanometer according to the ISO 81060-2, 2018 and ISO 81060-2 Amendment 1, 2020, which resulted in the currently pending Amendment to Amendment 1. J Clin Hypertens (Greenwich). 2022 Apr;24(4):513-518. Epub: 2022 Mar 21. doi: 10.1111/jch.14464. PMID: 35312160. Available from: PMC8989751.
81060-2:2018/Amd 1:2020 - Pass (n=85)
Note: This is a provisional list, as equivalence according to EU Regulation 2017/745 (e.g. MEDDEV 2.7/1 rev 4) is not proven. Accordingly, these publications are not used in the assessment of star-ratings.
A&D UA-1020
Zeng WF, Kang YY, Liu M, Li Y, Wang JG. Validation of the A&D UA-1020 upper-arm blood pressure monitor for home blood pressure monitoring according to the British Hypertension Society Protocol. Blood Press Monit. 2013 Jun;18(3):177-81. doi: 10.1097/MBP.0b013e32836141ca. PMID: 23571228.
BHS:1993 - Pass (A/A) With D-ring cuff
BHS:1993 - Pass (A/A) With cylindrical cuff
Zeng WF, Kang YY, Liu M, Li Y, Wang JG. Validation of the A&D UA-1020 upper-arm blood pressure monitor with six different-shaped or different-sized cuffs according to the British Hypertension Society protocol. Blood Press Monit. 2013 Oct;18(5):272-7. doi: 10.1097/MBP.0b013e32836464a3. PMID: 24013618.
BHS:1993 - Pass (A/A) General population
A&D UA-651 Plus (UA-656A-JCB1)
Alpert BS. Validation of the A&D UA-651 Plus/UA-651SL Plus automated sphygmomanometer according to the ISO 81060-2, 2018 and ISO 81060-2 Amendment 1, 2020, which resulted in the currently pending Amendment to Amendment 1. J Clin Hypertens (Greenwich). 2022 Apr;24(4):513-518. Epub: 2022 Mar 21. doi: 10.1111/jch.14464. PMID: 35312160. Available from: PMC8989751.
81060-2:2018/Amd 1:2020 - Pass (n=85)
